Medical Device Translation: Ensuring Patient Safety Across Global Markets
Imagine a life-saving defibrillator sitting idle in a Madrid hospital. Unused, because the medical staff can’t understand the badly translated instruction manual. Or imagine an insulin pump manufacturer facing a costly recall after translation errors in dosing instructions led to patient safety incidents across multiple European markets. Granted, these are hypothetical scenarios, but they represent very real risks that medical device companies face when translation quality falls short.
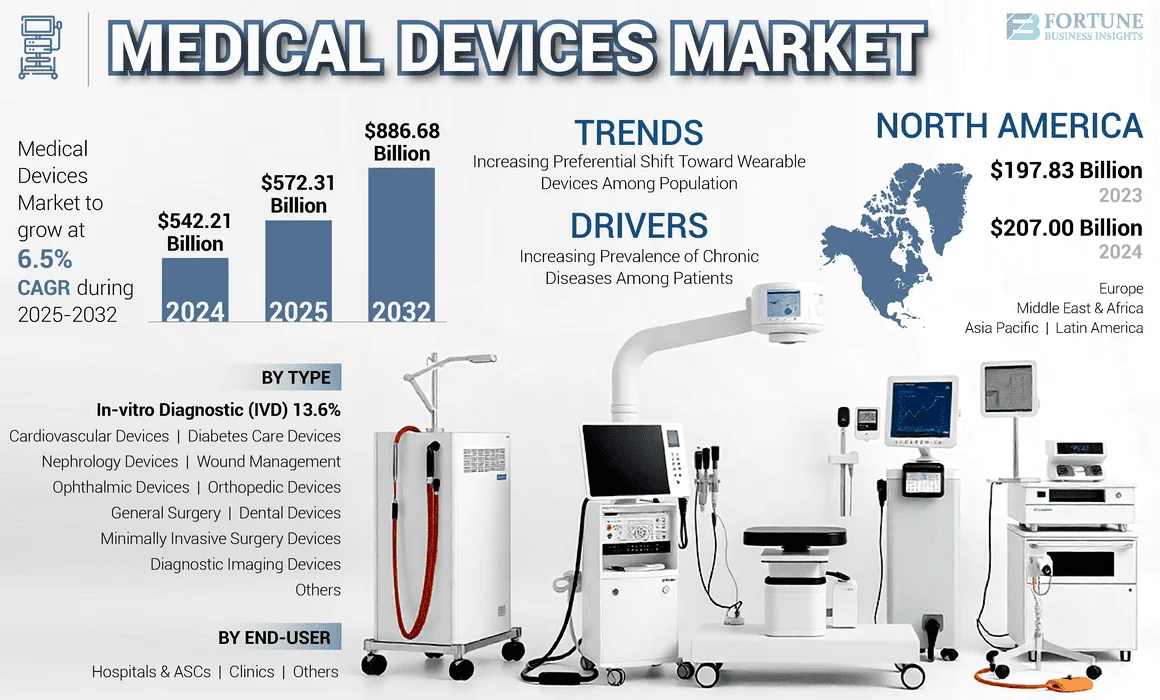
The global medical device market is worth over $540 billion (USD), which converts to a hefty £400 billion (GBP), with devices crossing borders daily to reach patients who desperately need them. But what many manufacturers don’t realise is that the difference between a successful global launch and a costly disaster often comes down to translation quality.
We feel confident you’d agree, when patient safety hangs in the balance, “good enough” simply isn’t good enough.
What can we help with today? Call us on +44 (0)1727 812 725 or email us at team@atlas-translations.co.uk – we’re only a call, chat, or email away, and we’re always eager to help!
The Hidden Risks of Poor Medical Device Translation
When Translation Mistakes Turn Dangerous
Medical device translation errors aren’t just embarrassing; they’re potentially lethal. We’ve read about cases where mistranslated dosage instructions led to overdoses and incorrectly translated warning labels resulted in device misuse.
The liability implications of poor translation, placing vulnerable patients at risk of contraindications, are staggering. Manufacturers face not only the immediate costs of product recalls and regulatory penalties but also long-term damage to their reputation and patient trust. In a far too connected world, news of translation-related safety issues spreads faster than you can say “product liability lawsuit.”
Beyond the Obvious: Cultural Considerations
Medical device translation travels far beyond converting words from one language to another. For one example, consider the complex nature of translating measurements. A ventilator setting in metric units can become dangerously confusing when improperly converted to imperial measurements for the US market.
Cultural attitudes towards medical procedures also vary dramatically. What’s considered routine in one country might require additional explanation or sensitivity in another. A device manual with a “cut and dry” approach in Germany might need significant cultural adaptation for markets in the Middle East or Asia.

Regulatory Compliance: Why Generic Translation Won’t Cut It
Navigating Global Regulatory Frameworks
Every major market has its own regulatory requirements, and the translation of medical device documentation must meet these specific standards. The FDA in the United States has strict guidelines for device labelling and instructions. European CE marking requirements demand precise translation of technical documentation and risk assessments. Health Canada, Australia’s TGA, and other regulatory bodies each have their own linguistic requirements.
For UK medical device companies, Brexit has added another layer of complexity. Devices that once enjoyed seamless European market access now require separate UK and EU regulatory submissions—each with its own translation requirements.
The Cost of Getting It Wrong
Regulatory rejections due to poor translation can delay market entry by months or even years. We’ve worked with clients who’ve faced resubmission costs exceeding £40,000 because their initial translations didn’t meet regulatory standards. Some manufacturers have had to conduct expensive product recalls when post-market research revealed translation-related safety issues.
The legal implications extend beyond immediate costs. Class action lawsuits, regulatory fines, and bad ties with distributors can damage a company’s profits for years.
The conversation about needing legal translation is something we’ll keep for another day. It’s safe to say that professional translation is also needed in this area.
What Makes Medical Device Translation Different
Technical Expertise Requirements
Medical device translation demands a unique combination of linguistic skill and technical knowledge. Translators must understand complex medical terminology across multiple languages, grasp the intricacies of regulatory language, and be familiar with international standards like ISO 17100 and ISO 13485.
It’s not enough to know that “contraindication” translates to “contraindicación” in Spanish—you can find that out with Google Translate. To go further, a qualified medical device translator understands the regulatory implications of that term and how it must be presented to meet local requirements.
Quality Assurance That Saves Lives
Professional medical device translation involves rigorous multi-stage quality assurance processes. Native speaker translators with medical backgrounds work alongside regulatory experts to make sure every document meets both linguistic and compliance standards. Consistency across all device documentation—from user manuals to quick start guides to packaging—is absolutely critical.
Version control becomes particularly important when devices are updated or modified. We maintain detailed translation memories to make sure that any changes to device specifications are accurately reflected across all translated materials, preventing dangerous inconsistencies.

Getting Your Medical Device Translation Right
What to Look for in a Translation Partner
When choosing a translation partner for medical devices, industry-specific experience isn’t just preferred—it’s essential. Look for agencies with proven track records in medical translation, relevant certifications, and deep knowledge of regulatory compliance across your target markets.
Turnaround times matter too, especially when you’re working towards strict launch deadlines. But speed should never come at the expense of quality when patient safety is at stake.
The Atlas Approach
At Atlas Translations, we understand that medical device translation is about more than words. We’ve been leading this industry for over 30 years, and we know it’s also about patient safety and business success. Our UK-based team combines an extensive medical translation network with a deep understanding of both domestic and international regulatory requirements.
We’ve helped medical device manufacturers navigate everything from simple user manual translations to complex regulatory submissions across multiple markets. Our quality-first approach means we never compromise on accuracy, even when deadlines are tight.
Summary
Medical device translation is a specialised field where precision isn’t just important, it’s a matter of life and death. Poor translation can lead to patient harm, regulatory rejections, costly recalls, and damaged reputations. The global nature of the medical device market means manufacturers must navigate complex regulatory frameworks whilst maintaining consistency across multiple languages and cultures.
The solution isn’t to cut corners on translation costs. We know it’s to partner with professionals who understand medical device translation through sector-specific knowledge. When patient safety and business success are both on the line, professional translation services aren’t just a wise investment—they’re an absolute necessity.
We’d love to hear from you!
You can call us on +44 (0)1727 812 725 or email team@atlas-translations.co.uk. We respond quickly to all enquiries and are always happy to chat about your needs.

If you’d like to visit us in person to learn more about our services or to drop off documents, just give us a call or email us to arrange a time.
During UK working hours, you can also use our Live Chat option (bottom right of the screen). You’ll always be connected with a real person, not a bot!
And if you need a fast estimate, our Get a Quote button at the top of the website makes it easy.
Can I Trust Atlas Translations?
Atlas Translations is certified to ISO 9001:2015 (Quality Management) and ISO 17100:2017 (Translation Services) standards. For confidential projects, we’re happy to sign a non-disclosure agreement (NDA).
We’ve been registered with the Information Commissioner’s Office (ICO) for over 20 years, reflecting our long-standing commitment to privacy and data protection.
We’re proud to provide fast, friendly, high-quality services—but don’t just take our word for it. Check out our client testimonials and TrustPilot reviews.
Global Voice, Local Touch
If you’re looking for some top tips for partnering with Atlas Translations, we have some top tips to share! We answer 25 of our clients’ most frequently asked questions, ranging from typesetting queries to discussing reference materials.
Click to download Global Voice, Local Touch
















